China is now vaccinating 3-year-olds in latest escalation of covid madness
10/29/2021 / By Zoey Sky
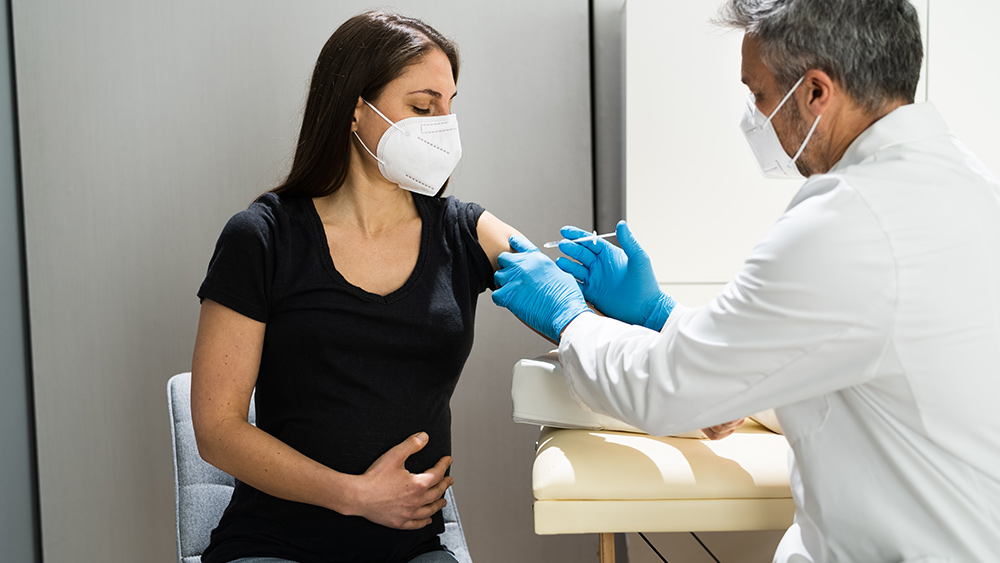
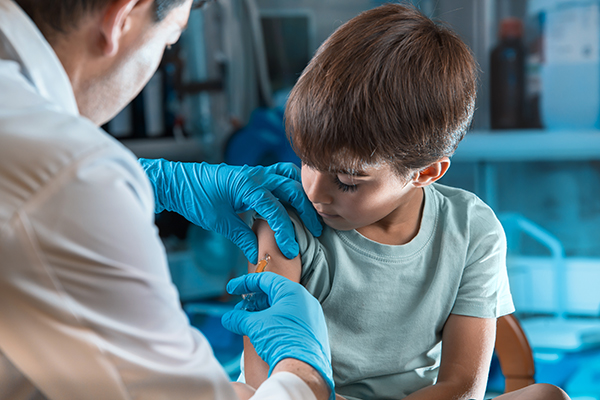
China has started to vaccinate children as young as three against the Wuhan coronavirus (COVID-19) to address more frequent outbreaks and the return of the delta variant.
According to local media, many areas throughout China are rolling out vaccines to children aged between three and 11. The vaccines come from two companies – Sinovac Biotech Ltd and Sinopharm.
Under China’s “zero-tolerance” COVID policy, borders are strictly monitored and citizens have given up their freedom as the government attempts to control outbreaks.
The policy entails shutting down borders to minimize imported cases. There are exceptions for residents, who can come and go if they are willing to endure a 21-day quarantine after returning to China. The policy also includes the complete lockdown of any location with confirmed signs of coronavirus cases.
The strict measures require participation from all citizens for it to work, with employees taking round-the-clock shifts to allow for constant testing of the whole population.
Social workers and medical staff in China help regulate individual lockdowns by patrolling the streets and regularly checking in on people undergoing quarantine. They also assist with food and grocery deliveries.
Citizens are also expected to stay at home and give up their freedom to ensure that the zero-COVID policy is effective. (Related: COVID-19 vaccine controversies continue amidst mandates and inoculation of children – Attorney Thomas Renz on Brighteon.TV.)
Back in June, China approved the use of coronavirus vaccines in children aged 12 and older. As of writing, the country has one of the highest vaccination rates among major economies.
To date, 75 percent of China’s 1.4 billion population are fully vaccinated. The country has also started implementing booster shots, with adults who already received their first dose of the vaccine now eligible.
Booster shots and vaccines for children are enforced as China tries to deal with the latest outbreak. Flareups of the coronavirus are more frequent now compared to before the spread of the delta variant.
But despite the setbacks, Beijing is committed to its zero-tolerance strategy. China’s capital kept borders closed and enforced quarantines even though other nations already started easing restrictions to gradually reopen economies and learn how to live with the virus.
Cases continue to spread in China
The latest delta outbreak quickly spread from the initial cluster in China’s northwest to at least one dozen provinces. China reported 35 locally-acquired coronavirus infections on Tuesday, Oct. 26, with four cases documented in Beijing.
Back in September, a cluster spread in southwestern Fujian province. The more contagious strain spread among elementary school students who were unvaccinated. Thankfully, many of the young patients only experienced mild symptoms.
Children are the last group to be targeted for vaccination, with approval for vaccines reconsidered amid the global spread of the delta variant. The variant prompted health authorities to either clear or consider rolling out shots.
According to a study, Pfizer-BioNTech’s mRNA vaccine is 91 percent effective at protecting children aged five to 11 from coronavirus. The U.S. plans to make vaccines available for the same age group, pending approval by the Food and Drug Administration (FDA).
Unlike the Pfizer-BioNTech vaccine, vaccines given to children in China use more traditional inactivated vaccine technology. The United Arab Emirates cleared Sinopharm’s vaccines for children as young as three back in August. In Argentina, children aged three to 12 are lining up for the Sinopharm vaccine.
Chile, which used Sinovac for a wider rollout, is now administering the same vaccine to kids aged six and older.
Despite the rollout of vaccines for younger children, public data on Sinovac’s efficacy in children is minimal. The company is planning a clinical trial for children in South Africa.
Sinovac started an efficacy trial with 14,000 children in several countries last September. However, Sinovac’s approval in China was only based on smaller phase 1 and phase 2 trials. Sinopharm’s Beijing vaccine was also approved based on smaller phase 1 and phase 2 trials, with findings eventually published in peer-reviewed journals.
But not all authorities are as lax on approving vaccines for children. Back in August, Brazil’s drug regulator rejected an application for the Sinovac vaccine to be used in minors because of the lack of recent data on the performance of the vaccine.
Visit Pandemic.news to learn how other countries are handling coronavirus infections among children.
Sources include:
Tagged Under: Big Pharma, children's health, China, covid-19, infections, medical fascism, Medical Tyranny, outbreak, pharmaceutical fraud, Sinopharm, Sinovac, Wuhan coronavirus, zero-Covid strategy
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 BIG PHARMA NEWS