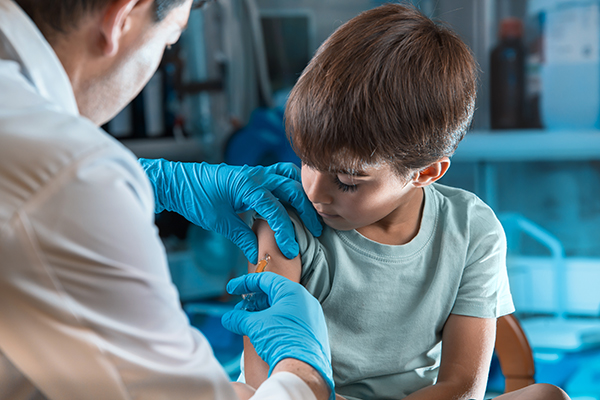
China is now offering coronavirus vaccines to toddlers as young as three
06/16/2021 / By Nolan Barton

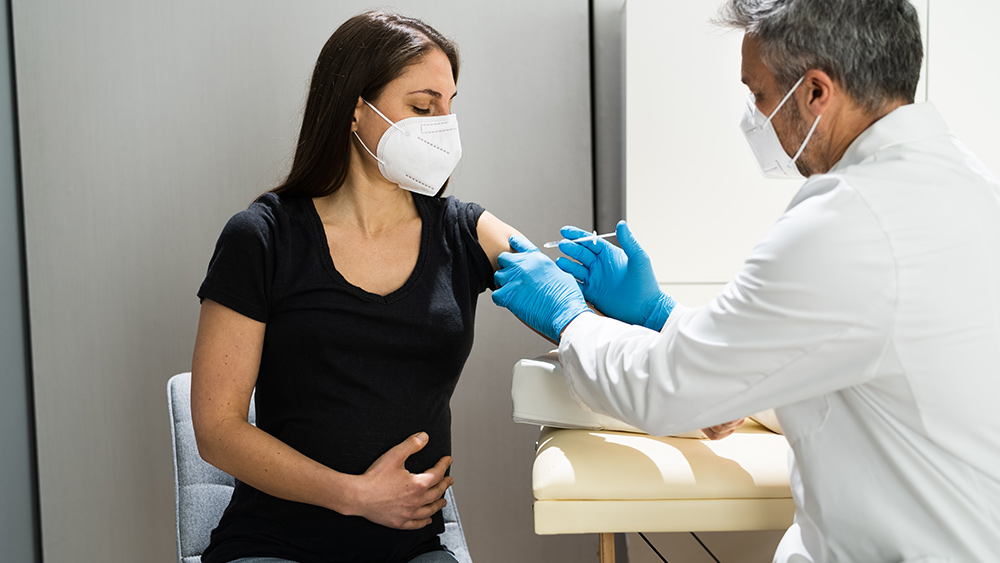
A spokesperson for Beijing-based vaccine maker Sinovac Biotech on Tuesday, June 8, confirmed that Chinese health authorities have approved the emergency use of the company’s coronavirus (COVID-19) vaccine for children as young as three years old.
“In recent days, the Sinovac vaccine was approved for emergency use in three- to 17-year-olds,” the spokesperson said.
Late last week, Sinovac Biotech Chief Executive Officer Yin Weidong told state broadcaster CCTV that “Sinovac has carried out a clinical study on the minor population, which started at the beginning of this year, with the first and second phase clinical trials completed.”
Yin noted that “hundreds of cases showed that after vaccination, the group [three to 17 years old] is as safe as the 18-year-old adult group.” (Related: SACRIFICE THE CHILDREN: Oxford Vaccine Group recruits children for coronavirus vaccine trials.)
A spokesperson for China’s other major vaccine, Sinopharm, said that experts had demonstrated the effectiveness of its vaccine in children, but did not confirm whether it had been approved for use.
WHO does not currently recommend vaccinating children
While the World Health Organization (WHO) does not currently recommend vaccinating children against coronavirus, the U.S., UK, Singapore and the European Union are now offering the Pfizer-BioNTech vaccine for children as young as 12. China is the first country to give emergency use authorization to COVID-19 vaccine for children below 12.
There are relatively few studies on the efficacy or potential side effects of the vaccines on children and they are regarded as a low priority compared with high-risk groups such as the elderly or frontline medical workers. Younger people generally thought to suffer from milder symptoms if infected with COVID-19.
It is not clear when the Chinese children are likely to start receiving COVID-19 vaccines as the country is still trying to meet a target of vaccinating 560 million people by the end of the month, with a particular focus on high-risk groups.
“The National Health Commission will organize relevant experts to promote the use of vaccines in lower age groups in an orderly manner based on the needs of the current epidemic in China and the composition of the population,” Yin said.
Chinese authorities claim new COVID variant affects all age groups
Chinese authorities said a new variant called delta, which was first identified in India, has caused an outbreak in the southern province of Guangdong. The variant is said to have nearly twice the viral load of previous strains and has affected all age groups. The youngest person infected was only one year old.
China reported 33 new COVID-19 cases on Tuesday, including 19 in Guangdong.
Yin said people in China and other parts of the world were increasingly willing to get vaccinated. But the increased demand also put pressure on supplies. China has mostly managed to bring the outbreak under control since the coronavirus first emerged in Wuhan. The country has administered more than 777 million vaccine doses after a sluggish start.
Expanding vaccination to children will inevitably put more pressure on vaccine producers. Yin said the designed capacity of Sinovac’s production plant was two billion doses a year but it was likely to exceed that in meeting demand from the domestic and international markets.
“We have now provided more than 600 million doses of vaccines to the world, which means that we have met the quality standards for providing vaccines to the WHO and more countries. We are prepared for further increases in production capacity,” Yin said.
Sinovac’s product is one of more than 20 vaccines developed in China using a variety of techniques, which range from using a dead, or inactivated, virus to trigger an immune response to using viral proteins or mRNA technology. An easy-to-use vaccine that can be sprayed in the mouth has also applied for emergency use, according to state media reports.
Follow Immunization.news for more news and information related to coronavirus vaccines.
Sources include:
Tagged Under: bad medicine, Big Pharma, China, coronavirus, covid vaccine, covid-19, covid-19 vaccines, dead virus, emergency use authorization, immune response, mRNA, mRNA technology, pandemic, viral proteins
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 BIG PHARMA NEWS