CDC asks governors to fast-track permits for coronavirus vaccine distribution
09/04/2020 / By Franz Walker

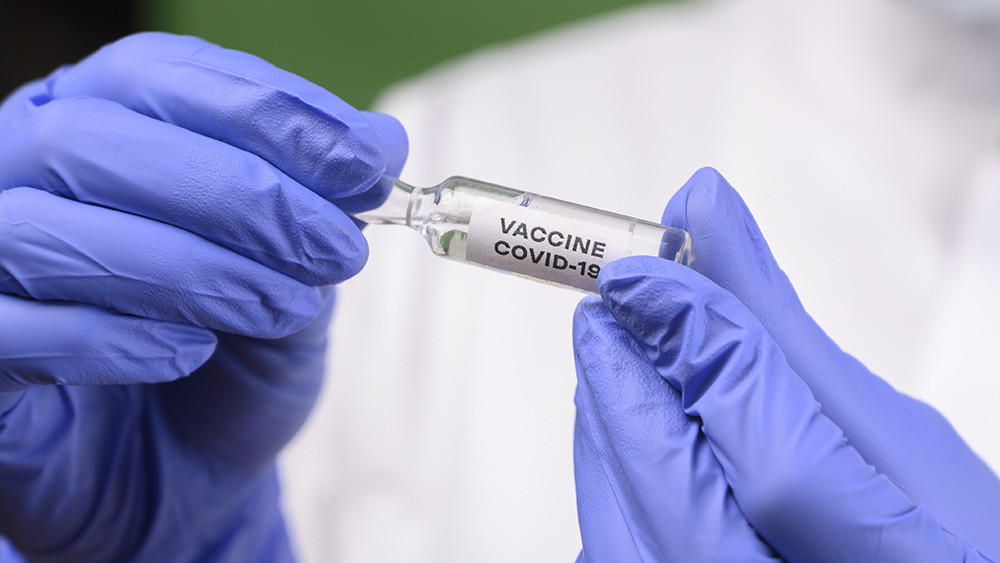
In a letter, dated August 27, the Centers for Disease Control and Prevention (CDC) is asking all state governors to fast-track permits to get a coronavirus vaccine out to the public by Nov. 1.
In the letter, which was obtained by McClatchy and reported on by the publishing company’s newspapers, CDC Director Dr. Robert Redfield requested that all governors fast-track permits and licenses for new distribution sites for a vaccine. He stated that the normal time that’s required to obtain these represented “a significant barrier to the success of this urgent public health program.”
“CDC urgently requests your assistance in expediting applications for these distribution facilities, and, if necessary, asks that you consider waiving requirements that would prevent these facilities from becoming fully operational by November 1, 2020,” Redfield wrote.
“The requirements you may be asked to waive in order to expedite vaccine distribution will not compromise the safety or integrity of the products being distributed,” he added.
President Trump “optimistic” that vaccine will hit before Election Day
President Donald Trump has previously stated that he was “optimistic” that a vaccine would be out, “in some cases,” before Nov. 3 – Election Day – on Geraldo Rivera’s radio program “Geraldo in Cleveland,” on WTAM on Aug. 6. He later reaffirmed this when asked about it on the White House South Lawn a couple of hours later.
“I’m optimistic that it’ll be probably around that date,” Trump said. “I believe we’ll have the vaccine before the end of the year, certainly, but around that date, yes, I think so.”
In addition to the CDC letter, delivery firms have also received guidance from Trump administration officials to prepare freezer farms in America’s heartland and get ready to load vaccines onto trucks no later than Nov. 1.
Already, shipping company UPS is building a giant freezer facility in Louisville, Kentucky, which can hold up to 48,000 vials of vaccine at temperatures as low as -112 F.
“This truly will be a historic supply chain feat to distribute millions, if not billions, of life-saving Covid-19 vaccine vials to far-reaching global populations,” said UPS Healthcare president Wes Wheeler. “Lives will depend on us to get these vaccine deployments right, and we’re well-prepared to support all of these efforts until this pandemic is behind us.”
The Atlanta-based courier company has been in discussion with the Department of Health and Human Services and the Operation Warp Speed team for a while now in regards to what is needed to distribute a vaccine.
FDA willing to waive Phase III trial requirement
At around the same time that the CDC sent out its letter, Food and Drug Administration (FDA) Commissioner Stephen Hahn also stated that his agency is willing to skip the critical Phase III trials for potential COVID-19 vaccines. (Related: You are a human guinea pig: FDA willing to let coronavirus vaccine makers SKIP crucial trial phase.)
“It is up to the sponsor [vaccine developer] to apply for authorization or approval, and we make an adjudication of their application. If they do that before the end of Phase Three, we may find that appropriate. We may find that inappropriate, we will make a determination,” said Hahn in an interview with Financial Times last Sunday.
While both initial trial phases only have vaccine candidates tested on smaller, more focused test groups, Phase III trials – the final trial stage – have it tested on thousands of people for efficacy and safety.
Hahn later clarified that the FDA would not technically approve any vaccine candidate that had not undergone Phase III trials. Instead, it would only issue what is called “emergency authorization” for its use.
“Our emergency use authorization is not the same as a full approval,” he explained. “The legal, medical and scientific standard for that is that the benefit outweighs the risk in a public health emergency.”
The efforts of the FDA and the CDC to speed up the deployment of a vaccine do raise questions if too many steps are being skipped to get them out. For one, Phase III trials are meant to catch possible issues with the vaccine that the more targetted Phase I and Phase II trials may have missed. Giving special “emergency authorization” to distribute a vaccine without the former means that the general public is in effect, acting as test subjects for what should have been the trial.
Meanwhile, fast-tracking permits for storage and distribution sites could risk some of these sites not to meet standards. Even if a vaccine works, it will still need to be carefully handled and stored in specific conditions – hence the reason UPS is building a special freezer farm for it.
Should any new facilities not meet the standards, just because they were fast-tracked, then it’s possible that any vaccines from these facilities may lose their efficacy or even become harmful to those who they’re given to.
Follow Vaccines.news for more on how many countries, and not just the U.S., are trying to fast-track their coronavirus vaccines.
Sources include:
Tagged Under: CDC, coronavirus, covid-19, disease, epidemic, FDA, Flu, immunization, outbreak, pandemic, President Donald Trump, superbugs, UPS, vaccine, vaccine wars, vaccines, virus
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 BIG PHARMA NEWS