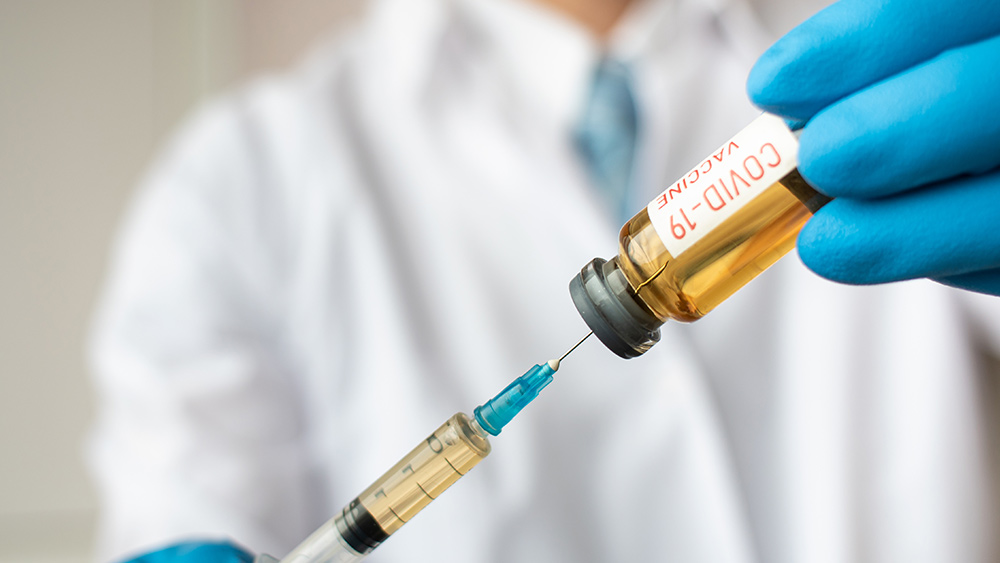
“We have a winner”: Trump administration inks $1.95-billion coronavirus vaccine deal, forcing taxpayers to enrich evil pharma giants
07/24/2020 / By Ralph Flores

The Trump administration will pay Pfizer and biotech firm BioNTech $1.9 billion for 100 million doses of their COVID-19 vaccine. If it proves safe and effective, these will be distributed free of charge to American citizens.
The agreement, the largest between the U.S. government and companies developing a coronavirus vaccine, also allows the U.S. to purchase further 500 million doses, the Department of Health and Human Services (HHS) said on Wednesday.
BioNTech and Pfizer are currently working on four potential vaccines. If the vaccines prove safe and effective in phase 3 trial – a large-scale testing phase for safety and efficacy – the HHS says that Pfizer will start to deliver vaccines across the country. The companies also say that they are looking to start a large phase 3 trial with up to 30,000 participants sometime this month.
President Donald Trump called the $1.95 billion deal a “historic agreement” during a press briefing on Wednesday, adding that soon, people can just into hospitals and get treated.
“Hopefully the approval process will go very quickly, and we think we have a winner there,” Trump added. “We also think we have other companies right behind that are doing very well on the vaccines, long ahead of schedule.”
The Trump administration is also working with state governments to ensure they have an adequate supply of remdesivir, the anti-viral drug touted to treat some coronavirus patients. (Related: Remdesivir shows “limited benefit” during trial, so why did the FDA approve it?)
The deal with Pfizer and BioNTech is the latest in the U.S. government’s Operation Warp Speed, an initiative by the federal government to deliver 300 million doses of a certified COVID-19 vaccine by January 2021. To date, it has already spent $1 billion on vaccines developed by Moderna and Johnson & Johnson and secured 300 million doses of another promising vaccine jointly developed by the University of Oxford and AstraZeneca.
BioNTech and Pfizer published the results of their clinical trial in the U.S. earlier this month. The results were promising, touted the company, as two dozen patients injected with the vaccine candidate showed immune responses that were similar or better than those in recovered COVID-19 patients.
“We made the early decision to begin clinical work and large-scale manufacturing at our own risk to ensure that product would be available immediately if our clinical trials prove successful,” added Pfizer CEO Albert Bourla.
Drug giant botched trials in the past
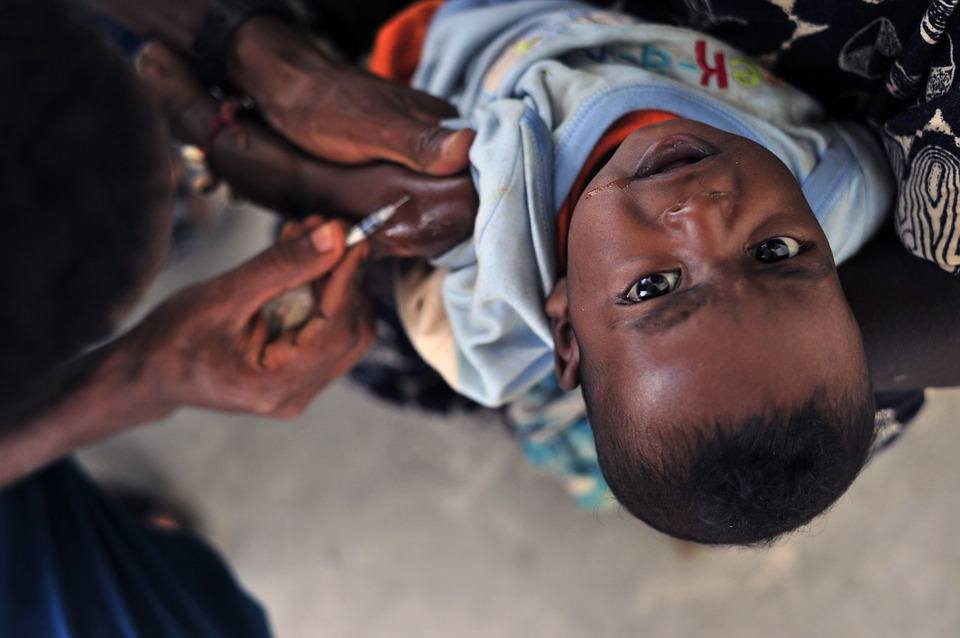
It’s worth noting at this point that Pfizer, the drug giant developing the vaccine, has landed in hot water for its questionable methods when it comes to drug trials. In 2011, the pharmaceutical company was ordered to pay $35 million in damages to the families of Nigerian children who had died or were severely harmed during a 1996 trial for an experimental drug. A total of 11 children died in the trial: five of them took the experimental drug Trovan, while the remaining after taking an older antibiotic in comparison. Other children suffered blindness, deafness and even brain damage.
The families of the children who participated in the trial also claimed that the drug giant did not have proper consent to use Trovan and that questions were raised over the documentation of the trial. For instance, some of the children received a dose lower than recommended, which left them with brain damage, slurred speech and paralysis.
Now, as the pharmaceutical company is tasked to develop another vaccine, some have raised their concerns on whether similar ethical concerns will appear in this trial. Another controversy that has dogged vaccine research is its practice of “tornado chasing,” or going where the disease is. The vaccine developed by Oxford and AstraZeneca is tested in Brazil and South Africa, where regulatory authorities are weaker and are prone to abuse and exploitation.
The U.S. now has over 4 million confirmed coronavirus cases and 144,242 deaths, according to data from Johns Hopkins University.
Learn more about the dark history of vaccine research at Vaccines.news.
Sources include:
Tagged Under: approval, Big Pharma, BioNTech, children, coronavirus, fast-track, FDA, guinea pigs, immunity, infections, medical experiments, Nigerian, outbreak, pandemic, Pfizer, vaccine, vaccine wars, vaccines
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 BIG PHARMA NEWS